Regain control of your GMP Documents.
All you need to manage your documents quickly and securely.

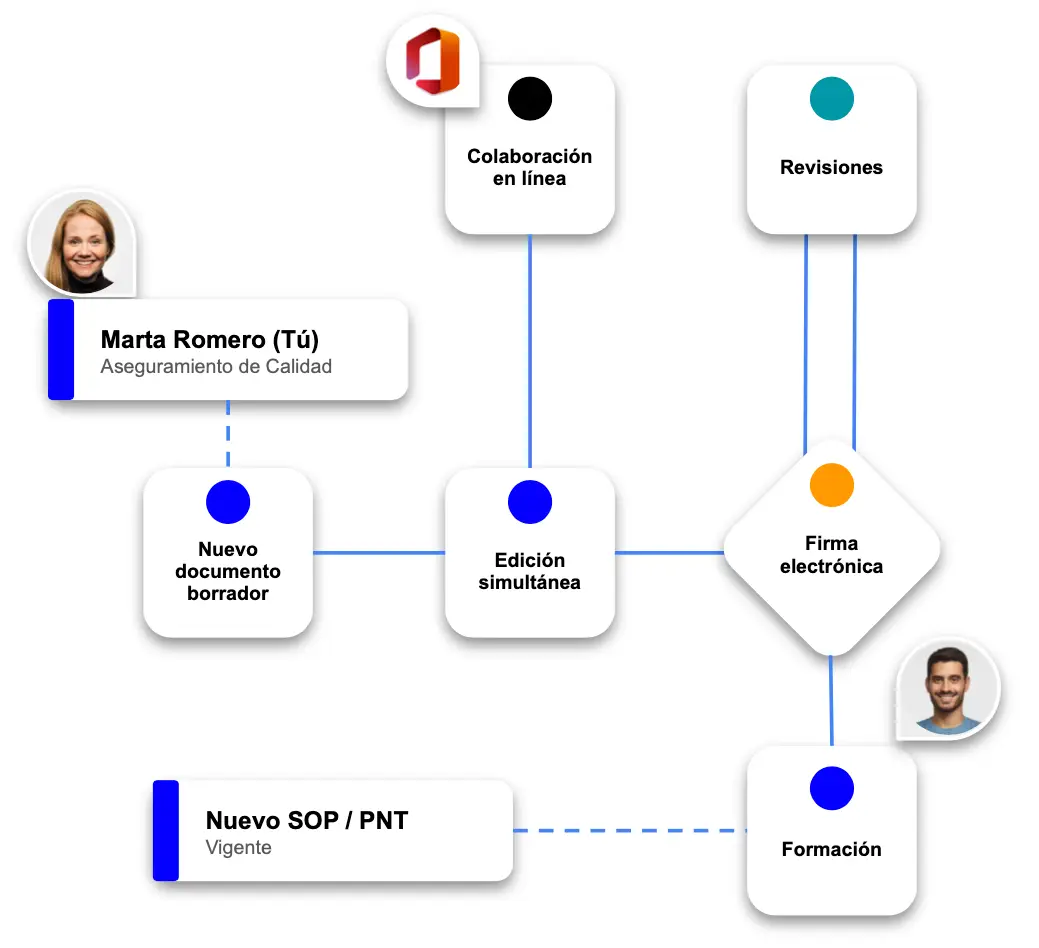
Document lifecycle management
Colaboration without friction.
Business effectiveness depends on the partnership
between people and teams from different areas.
Verifarma DMS Cloud offers all the resources to do it in
compliance with Regulations for Good Manufacturing Practice (GMP)
Teams committed to quality.
Adaptable workflows for document editing, collaboration and distribution in compliance with regulations.
Security
Reduced risk of deviations and loss of documents
Cohesion /Cooperation
Frictionless collaboration between the different areas of the company
Efficiency
Reduces time spent searching for and retrieving documents.
Control
Full traceability of all changes without information loss.
Reliability and adaptability
High quality documents
Automated workflows.
Notification system for an efficient collaboration between work teams.
Collaboration with external partners (CMOs, CROs, 3PLs).
Securely share your documents with business partners.
Integrated training system.
Create exams and training programmes by profile.
Digital signature and version control of each document.
Unlimited version control and auditing of changes.
Integration with MS Office 365
Simultaneous editing of documents for maximum speed.
Up to 1TB of storage per user.
Individual infrastructure per customer in European data centres.
Undertake transformation today
Get started, we'll help you.
You can rely on our implementation, migration, training and validation services.
- Ask for your personalised demo.
- We activate your environment and migrate your documents.
- We validate the system and start collaborating.
We empower both large and small companies
We empower both large and small companies



Request your demo
Register and we will contact you to schedule a personalised demo.
Our quality commitment
Ensuring the reliability of documents is a challenge.
- 12 years changing the pharmaceutical industry.
- Millions of documents managed.
- Hundreds of teams collaborating in the culture of quality

